Erikoislääkärit: Lopettakaa koronarokotukset
Erikoislääkärit Tamara Tuuminen ja Sylvi Silvennoinen-Kassinen ovat tänään lähettäneet hyvinvointialueiden valtuutetuille ao. kirjeen
(Via Ossi Tiihonen: X- postaus ja kirje)
KIRJE:
Arvoisa vastaanottaja,
Suomessa tarjotaan edelleen aktiivisesti koronarokotteita iäkkäille ihmisille. Nostamme tässä esille harvemmin julkisuudessa esiintuotuja seikkoja.
mRNA-KORONAINJEKTIOIDEN TEHOTTOMUUS
mRNA-koronainjektiot eivät täytä rokotteille asetettuja vaatimuksia. Tiedetään jo, että ne eivät suojaa rokotettua saamasta koronaa, eivätkä ne estä infektion leviämistä ihmisestä toiseen.
EU parlamentissa ryhmä MEPpejä on varmistanut yllä mainitun asian Euroopan lääkevirastolta (EMA). Sen on myös vahvistanut Pfizerin edustaja Janine Small. Hän on myöntänyt, ettei ole voitu osoittaa, että nämä mRNA-valmisteet estävät viruksen tarttumisen ihmisestä toiseen.
Kun selvisi, etteivät mRNA-koronainjektiot pystyneet estämään koronan leviämistä, tuli väite, että ne suojaavat vakavalta taudinkuvalta ja sairaalahoidolta. THL:n oma data osoittaa jo sen, että koronan takia sairaalahoitoon joutui ja kuoli sekä rokotettuja että rokottamattomia, ja vähitellen huomattavasti enemmän rokotettuja kuin rokottamattomia.
mRNA-KORONAINJEKTIOIDEN HAITALLISUUS
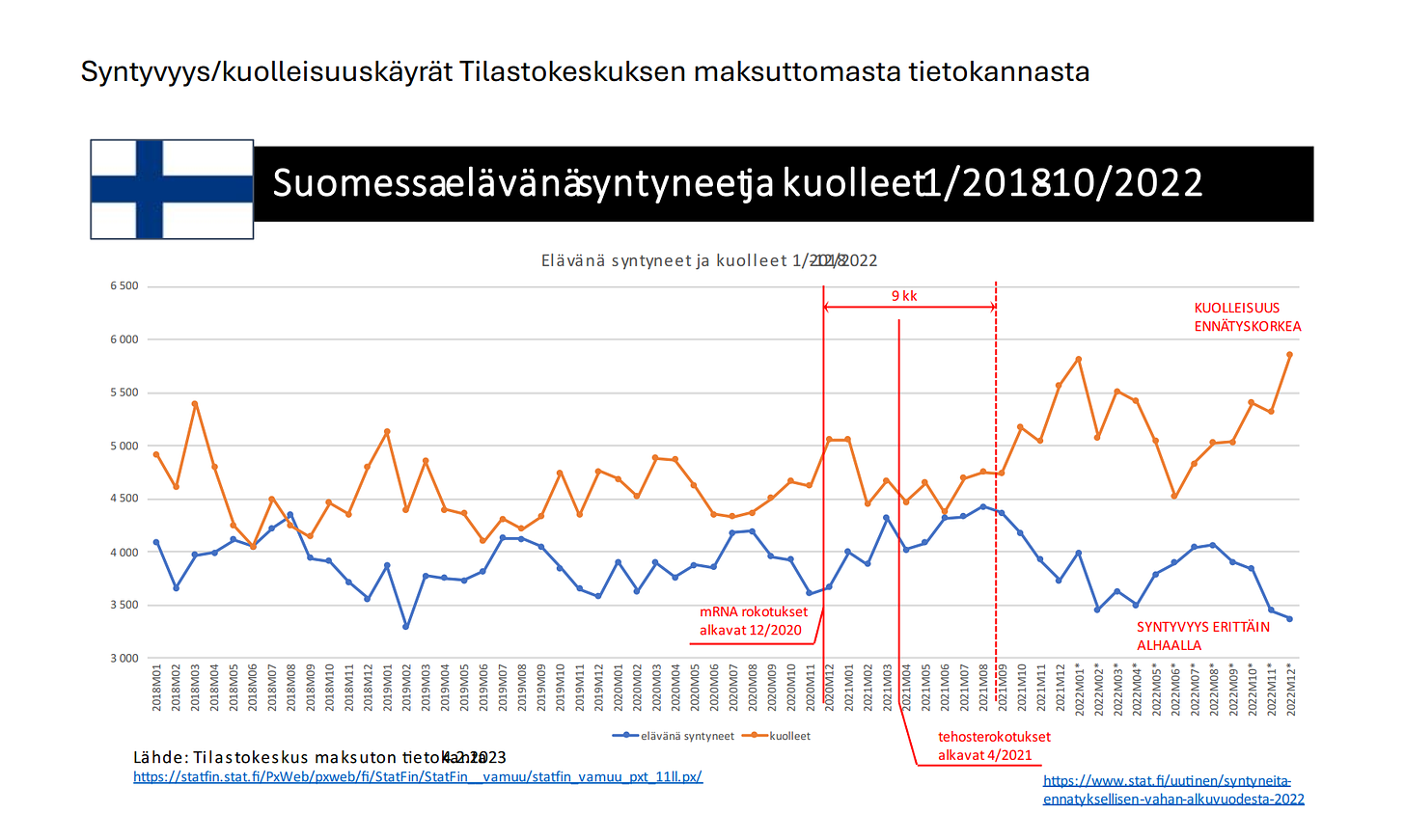
mRNA-koronainjektiot aiheuttavat tulehdusreaktioita, edistävät veritulppien muodostamista, heikentävät immuunivastetta ja edistävät ”turbosyöpien” syntyä. Lisäksi koronainjektioiden aloittamisesta on seurannut ylikuolleisuus, elinajan lyheneminen ja syntyvyyden romahdus.
VETOOMUS
Arvoisat luottamushenkilöt, tutustukaa esim. Pfizerin haittavaikutuslistaan, FIMEA:n haittavaikutustilastoon, ”turbosyöpien” tulvaan ja lisääntymiskyvyn heikkenemiseen.
Kun sallitaan tehottomat mRNA-koronainjektiot, jäävät jäljelle vain haitat. Tämä on ihmisten tietoista vahingoittamista ja rikos ihmisyyttä vastaan.
30 elokuuta, 2024
Kollegiaalisin terveisin
Tamara Tuuminen
kliinisen mikrobiologian erikoislääkäri lääketieteellisen mikrobiologian dosentti
Sylvi Silvennoinen-Kassinen
kliinisen mikrobiologian erikoislääkäri immunologian dosentti
Viitteet ja Liitteet
”Emme hyödy juurikaan koronarokotteesta. Siitä saa lyhytkestoisen, noin pari kuukautta kestävän ja ehkä 50 prosenttisen suojan tautia vastaan, mutta siihen saattaa liittyä riskejä, kun kovin tiuhaan rokotetaan”, kertoo Helsingin ja Uudenmaan sairaanhoitopiirin ylilääkäri Asko Järvinen:
https://www.mtvuutiset.fi/artikkeli/edessa-rankka-tautisyksy-laakarit-eivat-kuitenkaansuosittele-ylimaaraisia-rokotuksia-perusterveille/8993556
EU-parlamentaarikkojen vaatimus Euroopan lääkevirastolle (EMA) vetää mRNAkoronarokotteet pois markkinoilta: https://marceldegraaff.nl/wp-content/uploads/2023/10/Letter-suspension-marketingauthorizations-_231005_093400.pdf EMA:n vastaus EU-parlamentaarikoille: https://www.ema.europa.eu/en/documents/other/letter-members-parliament_.pdf EU-parlamentissa puhetilaisuus:
Chandler Robert W. (2023): Syntyvyyden laskua maailmassa: https://dailyclout.io/report-52-nine-months-post-covid-mrna-vaccine-rolloutsubstantial-birth-rate-drops/ Hagemann Raimund, Lorré Ulf, Kremer Hans-Joachim (2022): Syntyvyyden laskua Euroopassa: www.initiative-corona.info/fileadmin/dokumente/Geburtenrueckgang-Europe-EN.pdf
3
Syntyvyys/kuolleisuuskäyrät Tilastokeskuksen maksuttomasta tietokannasta
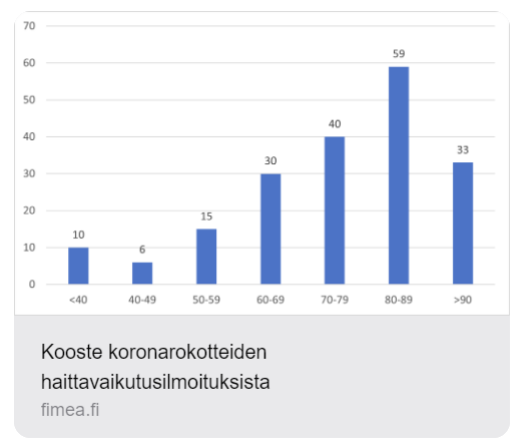
Pfizerin tiedossa olleet koronarokotehaitat.
https://x.com/myhiddenvalue/status/1822025871693377555
4
Linkki FIMEA:n tilastoihin haittavaikutuksista
https://dokumentit.neuvontapalvelu.net/Koronarokotteiden-haittavaikutusilmoitukset
Suomalainen tutkimus C19 rokotteiden haitoista
Tuuminen, T., Suominen, P. J., & Guldbrandsen, T. A. (2023). A Finnish Survey of Adverse Effects of COVID-19 Injectables and the Functionality of the Medical System. International Journal of Vaccine Theory, Practice, and Research, 3(1), 1009– 1025. https://doi.org/10.56098/ijvtpr.v3i1.87
Kansainväliset tutkimukset C19 rokotteiden haitoista
Koronarokotteiden vakavista haittavaikutuksista on jatkuvasti laajenevaa ja tarkentuvaa tutkimustietoa, kuten liitteenä olevasta artikkelien listasta voi havaita.
Yhteensä C19 rokotteiden aiheuttamia haittoja on raportoitu noin 3000 julkaisuissa. Alempana on pieni lista niistä.
Mörz, M. (2022). A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines, 10(10), Article 10. https://doi.org/10.3390/vaccines10101651
Mauro, V. P., & Chappell, S. A. (2014). A critical analysis of codon optimization in human therapeutics. Trends in Molecular Medicine, 20(11), 604– 613. https://doi.org/10.1016/j.molmed.2014.09.003
5
Ismail, I. I., & Salama, S. (2022). A systematic review of cases of CNS demyelination following COVID-19 vaccination. Journal of Neuroimmunology, 362, 577765. https://doi.org/10.1016/j.jneuroim.2021.577765
Santiago, D., & Oller, J. W. (2023). Abnormal Clots and All-Cause Mortality During the Pandemic Experiment: Five Doses of COVID-19 Vaccine Are Evidently Lethal to Nearly All Medicare Participants. International Journal of Vaccine Theory, Practice, and Research, 3(1), 847–890. https://doi.org/10.56098/ijvtpr.v3i1.73
Schreckenberg, R., Woitasky, N., Itani, N., Czech, L., Ferdinandy, P., & Schulz, R. (2024). Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. British Journal of Pharmacology, 181(3), 345–361. https://doi.org/10.1111/bph.16262
Eens, S., Van Hecke, M., Favere, K., Tousseyn, T., Guns, P.-J., Roskams, T., & Heidbuchel, H. (2023). B-cell lymphoblastic lymphoma following intravenous BNT162b2 mRNA booster in a BALB/c mouse: A case report. Frontiers in Oncology, 13, 1158124. https://doi.org/10.3389/fonc.2023.1158124
Polykretis, P., Donzelli, A., Lindsay, J. C., Wiseman, D., Kyriakopoulos, A. M., Mörz, M., Bellavite, P., Fukushima, M., Seneff, S., & McCullough, P. A. (2023). Autoimmune inflammatory reactions triggered by the COVID-19 genetic vaccines in terminally differentiated tissues. Autoimmunity, 56(1), 2259123. https://doi.org/10.1080/08916934.2023.2259123
Abou-Foul, A. K., Ross, E., Abou-Foul, M., & George, A. P. (2021). Cervical lymphadenopathy following coronavirus disease 2019 vaccine: clinical characteristics and implications for head and neck cancer services. The Journal of Laryngology and Otology, 135(11), 1025–1030. https://doi.org/10.1017/S0022215121002462
Effect of mRNA Vaccine Manufacturing Processes on Efficacy and Safety Still an Open Question. (2023). https://www.bmj.com/content/378/bmj.o1731/rr-2
Irrgang, P., Gerling, J., Kocher, K., Lapuente, D., Steininger, P., Habenicht, K., Wytopil, M., Beileke, S., Schäfer, S., Zhong, J., Ssebyatika, G., Krey, T., Falcone, V., Schülein, C., Peter, A. S., Nganou-Makamdop, K., Hengel, H., Held, J., Bogdan, C., … Tenbusch, M. (2022). Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Science Immunology, 8(79), eade2798. https://doi.org/10.1126/sciimmunol.ade2798
Kiszel, P., Sík, P., Miklós, J., Kajdácsi, E., Sinkovits, G., Cervenak, L., & Prohászka, Z. (2023). Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Scientific Reports, 13(1), Article 1. https://doi.org/10.1038/s41598-023-40103-x
6
Kumar, A., Narayan, R. K., Prasoon, P., Jha, R. K., Kumar, S., Kumari, C., Pandey, S. N., & Faiq, M. A. (2023). COVID-19 vaccination may enhance hippocampal neurogenesis in adults. Brain, Behavior, and Immunity, 107, 87– 89. https://doi.org/10.1016/j.bbi.2022.09.020
Perez, J.-C., & Foundation, L. M. (n.d.). Do Covid19 modified mRNA jabs Pose a Risk of Creating Harmful Proteins or Prions ?
Perez, J.-C., Moret-Chalmin, C., & Montagnier, L. (2023). Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection. International Journal of Vaccine Theory, Practice, and Research, 3(1), 727–770. https://doi.org/10.56098/ijvtpr.v3i1.66
Schreckenberg, R., Woitasky, N., Itani, N., Czech, L., Ferdinandy, P., & Schulz, R. (2024). Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. British Journal of Pharmacology, 181(3), 345–361. https://doi.org/10.1111/bph.16262
Segalla, G. (2023). Chemical-physical criticality and toxicological potential of lipid nanomaterials contained in a COVID-19 mRNA vaccine. International Journal of Vaccine Theory, Practice, and Research, 3, 787–817. https://doi.org/10.56098/ijvtpr.v3i1.68
Semmler, A., Mundorf, A. K., Kuechler, A. S., Schulze-Bosse, K., Heidecke, H., SchulzeForster, K., Schott, M., Uhrberg, M., Weinhold, S., Lackner, K. J., Pawlitzki, M., Meuth, S. G., Boege, F., & Ruhrländer, J. (2023). Chronic Fatigue and Dysautonomia following COVID-19 Vaccination Is Distinguished from Normal Vaccination Response by Altered Blood Markers. Vaccines, 11(11), 1642. https://doi.org/10.3390/vaccines11111642
Shastri, T., Randhawa, N., Aly, R., & Ghouse, M. (2022). Bone Marrow Suppression Secondary to the COVID-19 Booster Vaccine: A Case Report. Journal of Blood Medicine, 13, 69–74. https://doi.org/10.2147/JBM.S350290
Tokumasu, K., Fujita-Yamashita, M., Sunada, N., Sakurada, Y., Yamamoto, K., Nakano, Y., Matsuda, Y., Otsuka, Y., Hasegawa, T., Hagiya, H., Honda, H., & Otsuka, F. (2023). Characteristics of Persistent Symptoms Manifested after SARS-CoV-2 Vaccination: An Observational Retrospective Study in a Specialized Clinic for Vaccination-Related Adverse Events. Vaccines, 11(11), 1661. https://doi.org/10.3390/vaccines11111661
Yeni, M. (2023). COVID-19 BNT162b2 mRNA vaccine induced myocarditis with left ventricular thrombus in a young male. Acta Cardiologica, 78(4), 483– 485. https://doi.org/10.1080/00015385.2023.2165271
Alluqmani, M. (n.d.). New Onset Multiple Sclerosis Post-COVID-19 Vaccination and Correlation With Possible Predictors in a Case-Control Study. Cureus, 15(3), e36323. https://doi.org/10.7759/cureus.36323
7
Banoun, H. (2023). mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues. International Journal of Molecular Sciences, 24(13), Article 13. https://doi.org/10.3390/ijms241310514
Buergin, N., Lopez-Ayala, P., Hirsiger, J. R., Mueller, P., Median, D., Glarner, N., Rumora, K., Herrmann, T., Koechlin, L., Haaf, P., Rentsch, K., Battegay, M., Banderet, F., Berger, C. T., & Mueller, C. (n.d.). Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 booster vaccination. European Journal of Heart Failure, n/a(n/a). https://doi.org/10.1002/ejhf.2978
Chatterjee, A., & Chakravarty, A. (2023). Neurological Complications Following COVID- 19 Vaccination. Current Neurology and Neuroscience Reports, 23(1), 1– 14. https://doi.org/10.1007/s11910-022-01247-x
Chen, Y., Xu, Z., Wang, P., Li, X.-M., Shuai, Z.-W., Ye, D.-Q., & Pan, H.-F. (2022). Newonset autoimmune phenomena post-COVID-19 vaccination. Immunology, 165(4), 386– 401. https://doi.org/10.1111/imm.13443
Devaux, C. A., & Camoin-Jau, L. (2023). Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses, 15(5), Article 5. https://doi.org/10.3390/v15051045
Erdogan, M. A., Gurbuz, O., Bozkurt, M. F., & Erbas, O. (2024). Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations. Neurochemical Research. https://doi.org/10.1007/s11064-023-04089-2
Finsterer, J. (2022). Vaccine Adverse Event Reporting System Could Miss or Misinterpret Neurological Side Effects of COVID-19 Vaccinations. Annals of Neurology, 92(1), 157– 158. https://doi.org/10.1002/ana.26369
Frontera, J. A., Tamborska, A. A., Doheim, M. F., Garcia-Azorin, D., Gezegen, H., Guekht, A., Yusof Khan, A. H. K., Santacatterina, M., Sejvar, J., Thakur, K. T., Westenberg, E., Winkler, A. S., Beghi, E., & contributors from the Global COVID-19 Neuro Research Coalition. (2022). Neurological Events Reported after COVID-19 Vaccines: An Analysis of VAERS. Annals of Neurology, 91(6), 756–771. https://doi.org/10.1002/ana.26339
Garg, R. K., & Paliwal, V. K. (2022). Spectrum of neurological complications following COVID-19 vaccination. Neurological Sciences, 43(1), 3– 40. https://doi.org/10.1007/s10072-021-05662-9
Hertel, M., Heiland, M., Nahles, S., von Laffert, M., Mura, C., Bourne, P. E., Preissner, R., & Preissner, S. (2022). Real-world evidence from over one million COVID-19 vaccinations is consistent with reactivation of the varicella-zoster virus. Journal of the
8
European Academy of Dermatology and Venereology: JEADV, 36(8), 1342– 1348. https://doi.org/10.1111/jdv.18184
Høeg, T. B., Duriseti, R., & Prasad, V. (2023). Potential “Healthy Vaccinee Bias” in a Study of BNT162b2 Vaccine against Covid-19. The New England Journal of Medicine, 389(3), 284–285. https://doi.org/10.1056/NEJMc2306683
Huisman, W., Martina, B. E. E., Rimmelzwaan, G. F., Gruters, R. A., & Osterhaus, A. D. M. E. (2009). Vaccine-induced enhancement of viral infections. Vaccine, 27(4), 505– 512. https://doi.org/10.1016/j.vaccine.2008.10.087
König, B., & Kirchner, J. O. (2024). Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods and Protocols, 7(3), Article 3. https://doi.org/10.3390/mps7030041
Lu, L., Xiong, W., Mu, J., Zhang, Q., Zhang, H., Zou, L., Li, W., He, L., Sander, J. W., & Zhou, D. (2021). The potential neurological effect of the COVID‐19 vaccines: A review. Acta Neurologica Scandinavica, 144(1), 3–12. https://doi.org/10.1111/ane.13417
Nakatani, K., Sakata, E., Fujihara, M., Mizukawa, K., & Koyama, T. (2022). Systemic Vasculitis Following SARS-CoV-2 mRNA Vaccination Demonstrated on FDG PET/CT. Clinical Nuclear Medicine, 47(5), e403– e405. https://doi.org/10.1097/RLU.0000000000004115
Parry, P. I., Lefringhausen, A., Turni, C., Neil, C. J., Cosford, R., Hudson, N. J., & Gillespie, J. (2023). ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines, 11(8), Article 8. https://doi.org/10.3390/biomedicines11082287
Patone, M., Handunnetthi, L., Saatci, D., Pan, J., Katikireddi, S. V., Razvi, S., Hunt, D., Mei, X. W., Dixon, S., Zaccardi, F., Khunti, K., Watkinson, P., Coupland, C. A. C., Doidge, J., Harrison, D. A., Ravanan, R., Sheikh, A., Robertson, C., & Hippisley-Cox, J. (2021). Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nature Medicine, 27(12), Article 12. https://doi.org/10.1038/s41591-021- 01556-7
Pujol, A., Gómez, L.-A., Gallegos, C., Nicolau, J., Sanchís, P., González-Freire, M., LópezGonzález, Á. A., Dotres, K., & Masmiquel, L. (2022). Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves’ disease to silent thyroiditis. Journal of Endocrinological Investigation, 45(4), 875–882. https://doi.org/10.1007/s40618-021-01707-0
Riad, A., Põld, A., Kateeb, E., & Attia, S. (2022). Oral Adverse Events Following COVID-19 Vaccination: Analysis of VAERS Reports. Frontiers in Public Health, 10. https://www.frontiersin.org/articles/10.3389/fpubh.2022.952781
9
Santiago, D. (2022). Playing Russian Roulette with Every COVID-19 Injection: The Deadly Global Game. International Journal of Vaccine Theory, Practice, and Research, 2(2), 619–650. https://doi.org/10.56098/ijvtpr.v2i2.36
Shaw, C. (2020). Weaponizing the Peer Review System. International Journal of Vaccine Theory, Practice, and Research, 1(1), 11–26. https://doi.org/10.56098/ijvtpr.v1i1.1
Snapiri, O., Rosenberg Danziger, C., Shirman, N., Weissbach, A., Lowenthal, A., Ayalon, I., Adam, D., Yarden-Bilavsky, H., & Bilavsky, E. (2021). Transient Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19 Vaccine. The Pediatric Infectious Disease Journal, 40(10), e360–e363. https://doi.org/10.1097/INF.0000000000003235
Starekova, J., Bluemke, D. A., Bradham, W. S., Grist, T. M., Schiebler, M. L., & Reeder, S. B. (2021). Myocarditis Associated with mRNA COVID-19 Vaccination. Radiology, 301(2), E409–E411. https://doi.org/10.1148/radiol.2021211430
Terentes-Printzios, D., Gardikioti, V., Solomou, E., Emmanouil, E., Gourgouli, I., Xydis, P., Christopoulou, G., Georgakopoulos, C., Dima, I., Miliou, A., Lazaros, G., Pirounaki, M., Tsioufis, K., & Vlachopoulos, C. (2022). The effect of an mRNA vaccine against COVID- 19 on endothelial function and arterial stiffness. Hypertension Research, 45(5), Article 5. https://doi.org/10.1038/s41440-022-00876-6
Tinari, S. (2021). The EMA covid-19 data leak, and what it tells us about mRNA instability. BMJ, 372, n627. https://doi.org/10.1136/bmj.n627
Umei, T. C., Kishino, Y., Watanabe, K., Shiraishi, Y., Inohara, T., Yuasa, S., & Fukuda, K. (2022). Recurrence of Myopericarditis Following mRNA COVID-19 Vaccination in a Male Adolescent. CJC Open, 4(3), 350–352. https://doi.org/10.1016/j.cjco.2021.12.002
Vidula, M. K., Ambrose, M., Glassberg, H., Chokshi, N., Chen, T., Ferrari, V. A., & Han, Y. (2021). Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus, 13(6), e15576. https://doi.org/10.7759/cureus.15576
Watkins, K., Griffin, G., Septaric, K., & Simon, E. L. (2021). Myocarditis after BNT162b2 vaccination in a healthy male. The American Journal of Emergency Medicine, 50, 815.e1-815.e2. https://doi.org/10.1016/j.ajem.2021.06.051
Welsh, K. J., Baumblatt, J., Chege, W., Goud, R., & Nair, N. (2021). Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine, 39(25), 3329–3332. https://doi.org/10.1016/j.vaccine.2021.04.054
White, E., Fazio, N., Tourmouzis, K., Ryu, S., Finger, P. T., Sassoon, J., Keresztes, R., Chou, T., Kaplowitz, K., & Honkanen, R. (2024). Unilateral conjunctival Classic Kaposi Sarcoma following a COVID 19 booster. American Journal of Ophthalmology Case Reports, 34, 101986. https://doi.org/10.1016/j.ajoc.2023.101986
10
Zelkoski, A. E., Ennis, K. H. E., Lu, Z., & Malloy, A. M. W. (2023). The BNT162b2 vaccine’s empty lipid nanoparticle is able to induce an NF-κB response. The Journal of Immunology, 210(1_Supplement), 160.18. https://doi.org/10.4049/jimmunol.210.Supp.160.18
Abou-Foul, A. K., Ross, E., Abou-Foul, M., & George, A. P. (2021). Cervical lymphadenopathy following coronavirus disease 2019 vaccine: clinical characteristics and implications for head and neck cancer services. The Journal of Laryngology and Otology, 135(11), 1025–1030. https://doi.org/10.1017/S0022215121002462
Hou, Y., Zhao, J., Martin, W., Kallianpur, A., Chung, M. K., Jehi, L., Sharifi, N., Erzurum, S., Eng, C., & Cheng, F. (2020). New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Medicine, 18(1), 216. https://doi.org/10.1186/s12916-020-01673-z
Jahankhani, K., Ahangari, F., Adcock, I. M., & Mortaz, E. (2023). Possible cancer-causing capacity of COVID-19: Is SARS-CoV-2 an oncogenic agent? Biochimie, 213, 130– 138. https://doi.org/10.1016/j.biochi.2023.05.014
Perez, J.-C. (n.d.). Are Covid19 mRNA Injections the Cause of Turbo Cancers due to Prion Behavior of P53 Tumor Suppressor?
Uversky, V. N., Redwan, E. M., Makis, W., & Rubio-Casillas, A. (2023). IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines, 11(5), Article 5. https://doi.org/10.3390/vaccines11050991
White, E., Fazio, N., Tourmouzis, K., Ryu, S., Finger, P. T., Sassoon, J., Keresztes, R., Chou, T., Kaplowitz, K., & Honkanen, R. (2024). Unilateral conjunctival Classic Kaposi Sarcoma following a COVID 19 booster. American Journal of Ophthalmology Case Reports, 34, 101986. https://doi.org/10.1016/j.ajoc.2023.101986
11
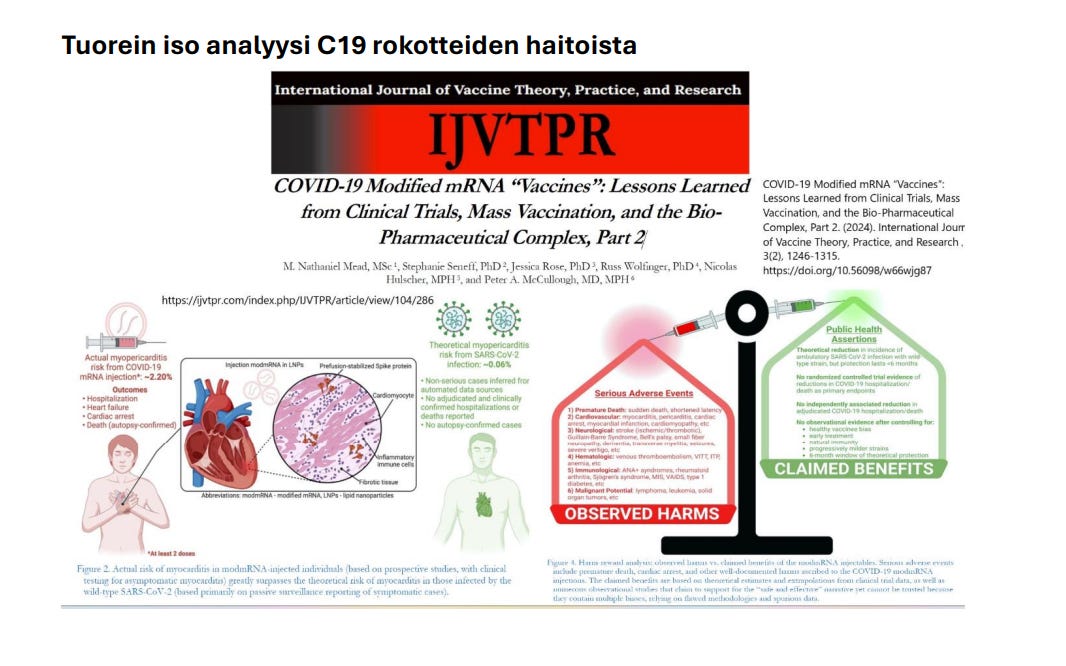
Tuorein iso analyysi C19 rokotteiden haitoista